OliPass PNA
Geared up with excellent cell permeability and strong
affinity for RNA, OliPass PNA is self-evolving as the most ideal platform technology.
Geared up with excellent cell
permeability and strong affinity for
RNA, OliPass PNA is self-evolving as
the most ideal platform technology.
Conventional Antisense Oligonucleotides
Antisense therapeutic involves the delivery of an artificial nucleic acids into the cell and binding to the specific region of a pre-mRNA or mRNA to inhibit translation of the disease-causing protein. For the past 50 years, scientists have tried to modulate RNA functions with artificial nucleic acids. To date, phosphorothioate, 2-alkyloxy RNA, morpholino, locked nucleic acid, and siRNA are artificial nucleic acids popularly investigated for therapeutic purposes. Since the poor cell permeability of such artificial nucleic acids, there are essentially no successful cases of RNA modulating drugs with practical applicability.
PNA
Peptide nucleic acid (PNA) is a novel class of artificial nucleic acids invented by Dr. Nielson and coworkers in 1990. Despite its attractive properties as nucleic acid resembling natural DNA or RNA oligonucleotide, PNA has never been developed as therapeutic agents due to its poor cell permeability and physicochemical properties.
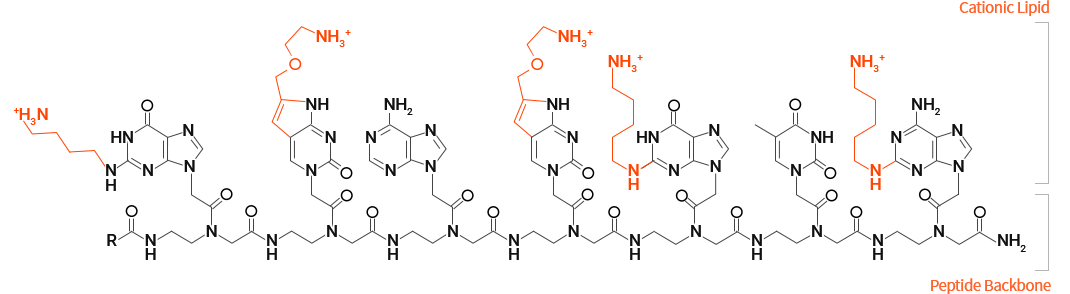
OliPass PNA
OliPass Peptide Nucleic Acid (OliPass PNA) was derived from PNA by rationally introducing cationic lipid moiety onto nucleobase. By covalently attaching such cationic lipid groups onto PNA, the cell permeability markedly improved. In addition, the affinity for RNA increased by at least a million times. Geared up with excellent membrane permeability and ultra-strong affinity for RNA, OliPass PNA is self-evolving as the most ideal platform for artificial nucleic acid therapeutics.
OliPass PNA Mode of Action
Due to its potential to readily pass through the cell membrane, OliPass PNA is an artificial nucleic acids suitable for modulating the splicing process inside a nucleus. In addition, since OliPass PNA tightly and selectively binds to the pre-mRNA to prevent spliceosome complex formation, it effectively induces exon skipping at concentrations as low as billion times that of other class of artificial nucleic acids. Like other artificial nucleic acids, OliPass PNA can also bind to the mRNA and inhibit protein synthesis. However the concentration of OliPass PNA to work on mRNA is billion times higher than on pre-mRNA, so the mechanism of action on mRNA would not be meaningful from a therapeutic perspective.
OliPass PNA Pros and Cons
-
Excellent therapeutic efficacy was observed in animals administered with 10 ng/Kg, about 100,000 times less dose concentration than the dose of other conventional artificial nucleic acids therapeutic drugs. The OliPass PNA therapy with very low therapeutic dose concentration can be supplied to patients at reasonably low price since API manufacturing cost burden is minimal.
OliPass PNA is expected to be applied to the development of most of the therapeutic agents as an innovative drug development platform considering the fact that such excellent safety and realistic drug prices are not presented in the field of conventional artificial nucleic acids therapy due to technical limitations.
-
Because OliPass PNA is distributed evenly throughout the body, it can be developed for treating most diseases such as cancer, diabetes, Alzheimer’s disease, Duchenne Muscular Dystrophy, Parkinson’s disease, chronic pain, rheumatoid arthritis, gout, cirrhosis and pulmonary fibrosis.
-
OliPass PNA is highly permeable to skin, so it is also suitable to be developed for treating various skin diseases such as atopic dermatitis, psoriasis and the like. Also due to its excellent skin permeability, OliPass PNA is suitable for development for life style management such as wrinkle improvement, hair loss prevention, whitening, obesity and cellulite removal.
-
As OliPass PNA is also easily delivered into the eyeball with topical eye drop administration, various retinal diseases such as age-related macular degeneration (AMD), diabetic retinopathy, glaucoma and visual loss can be treated with convenient formulation. Considering the intimidating reality of injecting a needle into the eyeball for retinal treatment, OliPass PNA can provide outstanding ease of use, compliance and safety to the patient population.
-
OliPass PNA may not have an excellent level of oral absorption, but it exhibits oral dosing efficacy sufficient for development as a therapeutic for liver or digestive system diseases. Therefore, it is expected that it will be possible to effectively and safely control diseases such as hyperlipidemia, fatty liver, liver cirrhosis, and chronic enteritis when it is developed as an oral drug instead of an injection.
| Patent Number | Patent Title | Registered Country |
|---|---|---|
| 10-2019-0054681 | Matrixmetalloproteinase-1 Antisense Oligonucleotides | South Korea |
| 10-2018-0095124 | Acetyl-CoA Carboxylase2 Antisense Oligonucleotides | South Korea |
| PCT/IB2017/000697 | Androgen Receptor Antisense Oligonucleotides | PCT |
| PCT/KR2018/008143 | Tyrosinase Antisense Oligonucleotide | South Korea, 3 foreign countries |
| PCT/IB2017/001385 | HIF1-a Antisense Oligonucleotide | PCT |
| PCT/IB2017/000751 | SCN9A Antisense Oligonucleotide | PCT |
| PCT/IB2017/001725 | Exon Skipping by Peptide Nucleic Acid Derivatives | PCT |
| PCT/KR2009/001256 | Peptide Nucleic Acid Derivatives with Good Cell Penetration and Strong Affinity for Nucleic Acid | South Korea, 37 foreign countries |